ABS-201™ for Endometriosis
Overview
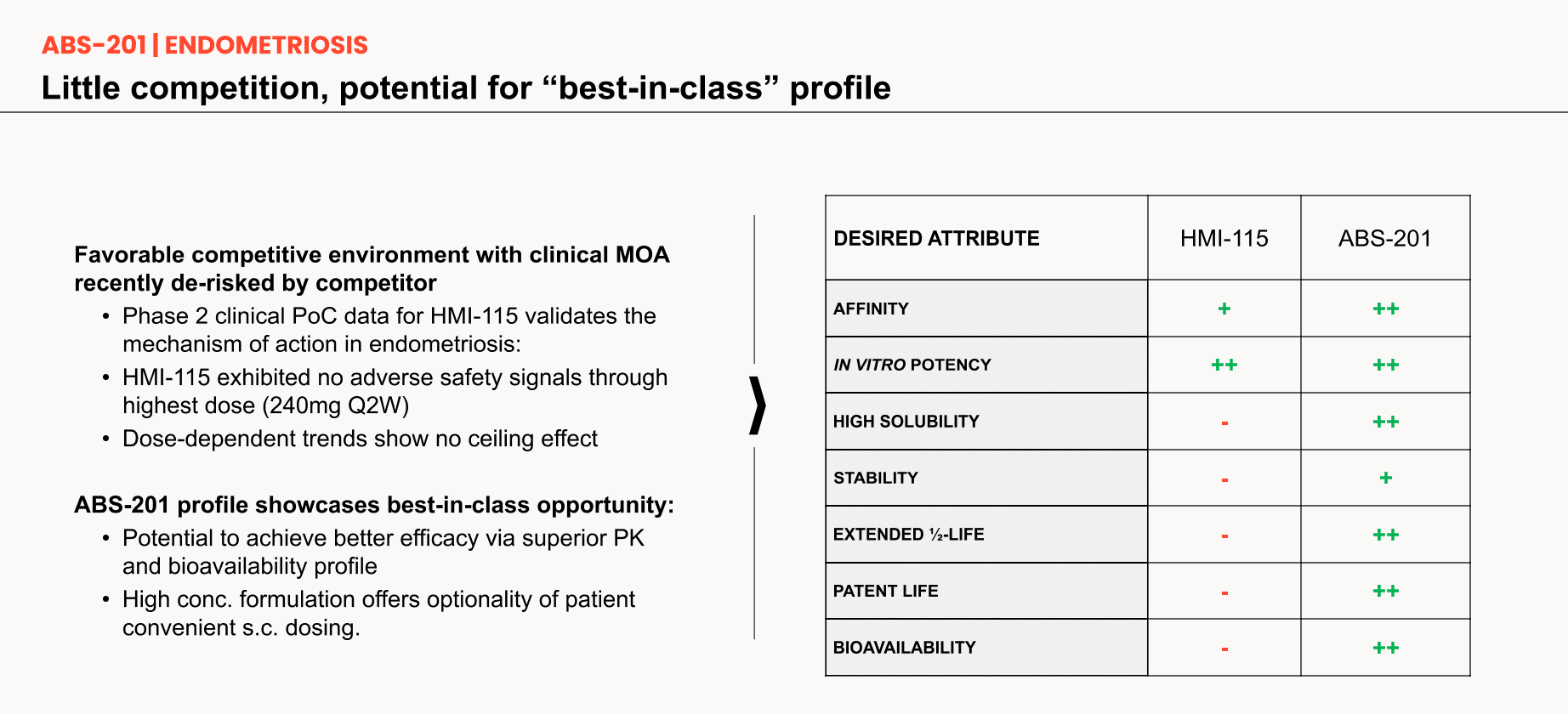
Potential best-in-class anti-prolactin receptor antibody for endometriosis, positioned to offer a uniquely differentiated mechanism of action
Currently in Phase 1, with endometriosis Phase 2 initiation projected for Q4 2026 and an interim data readout anticipated 2027
- High affinity and potency
- Non-sex-steroid hormonal MOA
- Potential dual action on pain pathway and lesion proliferation
- Strong developability profile
- Validated (de-risked) mechanism
- Anticipated low immunogenicity
- Extended half-life resulting in potentially longer dosing intervals for patient convenience
Unmet Need for Advancements in Treatment
Endometriosis is a chronic, painful, inflammatory condition that affects one in 10 women of reproductive age in the U.S. This indication has a significant unmet need and is defined by the formation of endometrial-like tissue and lesions outside the uterus that can cause inflammation, ovarian cysts, adhesions, scar tissue, and subsequent infertility. The symptoms include pelvic pain (∼80–90%), heavy bleeding (∼60%), infertility (∼30%), and ovarian cysts (∼20%). This disease is also associated with impaired quality of life and daily functioning, anemia, sleep disturbances, and fatigue.
Current medical and surgical treatments for endometriosis are primarily aimed on symptom management
The standard of care focuses on managing pain symptoms, controlling estrogen stimulation, and modulating the menstrual cycle. Hormonal birth control, GnRH therapies, and over-the-counter pain medications are commonly prescribed for patients with endometriosis. However, none of the FDA-approved treatments address the underlying disease pathway. Up to 33% of patients do not respond to hormonal treatment alone and many patients attempting to conceive require treatment adjustment or cessation to support pregnancy.

Targeting PRLR
By blocking prolactin receptors, ABS-201 may modify the underlying pathogenesis of endometriosis, offering dual action in reducing overall lesion development and pain.
Prolactin is overexpressed in the endometrium of patients with endometriosis. Within endometriotic lesions, prolactin promotes both tissue lesion growth and the hypersensitivity of pain-sensing nerves contributing to the chronic and acute pelvic pain experienced during menstruation and intermenstrual.
ABS-201's mechanism of action is validated by both internal and external pre-clinical data, as well as promising clinical results from HMI-115 that showed efficacy toward reducing pain in endometriosis patients. This derisked foundation positions ABS-201 as a potential best in class therapeutic.

Preclinical profile
The mechanism of ABS-201 for endometriosis is established in several preclinical studies. Preclinical studies suggest prolactin receptor antagonism suppresses postoperative pain in female mice and inhibits endometriosis interna formation.
The novel, nociceptor-specific mechanism for prolactin signaling in regulating pain responses, particularly in females, was identified in a study of male and female adult mice. Administering a prolactin receptor antagonist to the mice’s hind paw or spinal cord substantially reduced pain sensitivity in models of inflammation. Another preclinical study in female mice with endometriosis showed that prolactin antagonism significantly reduced lesion formation, suggesting disease-modifying effects beyond just symptom control.
IND-enabling studies completed for ABS-201
ABS-201 is currently in a Phase 1 clinical trial